B.R.A.I.N. Lab Research
B.R.A.I.N. Lab Research Projects
Constrained Hemisphere Aphasia Therapy (CHAT)
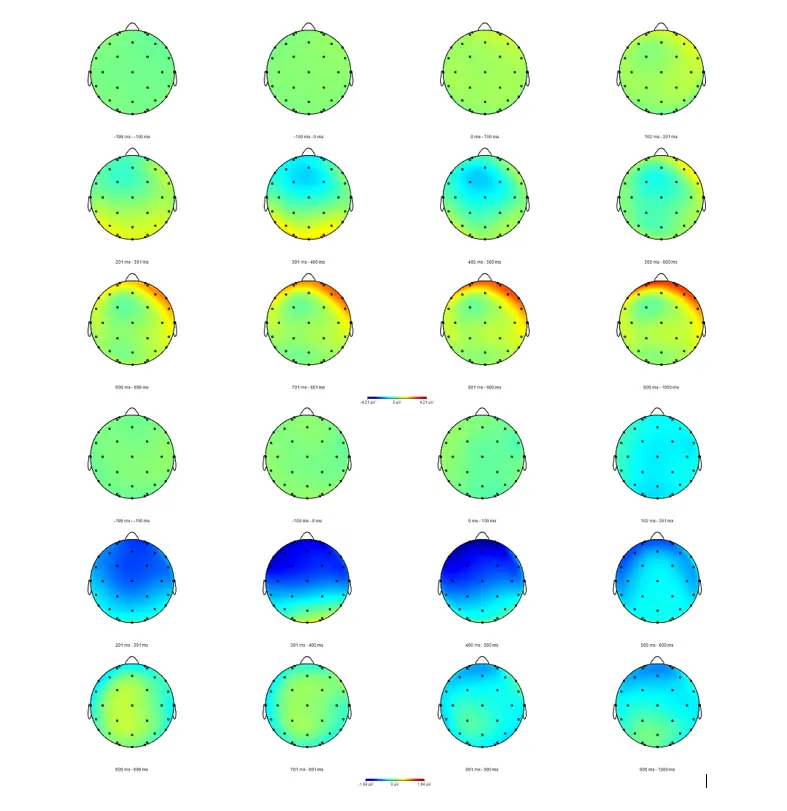
In the first, pilot, study we are examining whether the CHAT protocol can shift processing of primary language functions (phonology, morphology, syntax, and semantics) to the right hemisphere in non-injured adults.
So far, 12 men and 19 women have completed the study. To the left are their post-training minus pre-training topographical difference maps; women's results in the first 3 rows, and men's results in the bottom 3 rows.
The second study, currently in design, will implement the CHAT protocol in a single-subject A-B-A design to determine whether or not CHAT can elicit improved language functions in chronic severe aphasia by shifting primary language processing to the right hemisphere.
Downstream projects will seek to determine which would better facilitate language improvement for post stroke aphasia in general – targeting the hemisphere containing the lesion, or the contralateral (non-injured) hemisphere; this may depend on the size, location, and severity of the stroke.
This research is made possible by generous research grants for faculty (California State University, East Bay, Faculty Support Grant) and students (California State University, East Bay, Center for Student Research, Cal State East Bay; Texas State University, Research Enhancement Program).
Cognitive Rehabilitation Therapy (CRT) for Traumatic Brain Injury
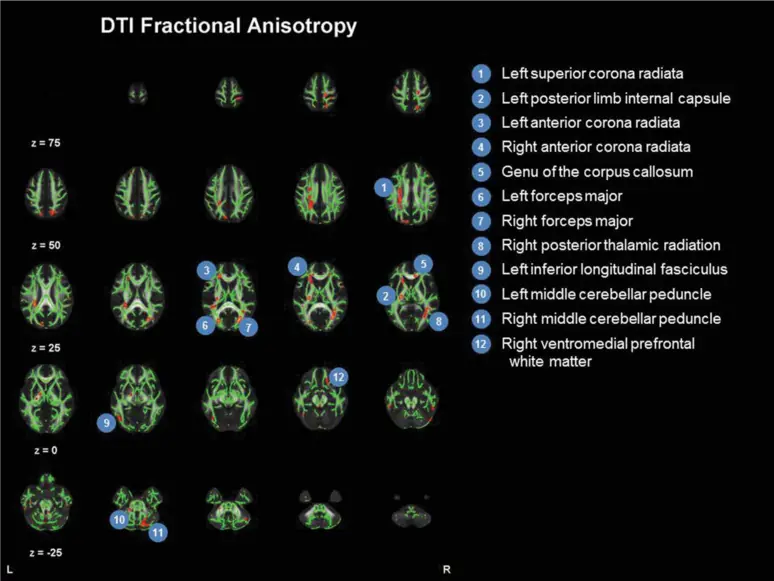
There is a great need for clinical research in the area of cognitive rehabilitation treatment (CRT) for the cognitive impairments that are commonly experienced after Traumatic Brain Injury (TBI), particularly during the chronic phase of recovery. Therefore, we are investigating the ways in which evidence-based CRT protocols affect brain structure and function, reflecting treatment-induced neuroplasticity in brain networks that are believed to subserve cognitive processes such as attention, memory, and executive function.
For the initial single case study, see Ramanathan, Turner, & Stevens (2019). This study constituted Heather Turner’s master’s thesis at the University of Connecticut (UConn), and was conducted in partnership with Dr. Michael C. Stevens, Olin Neuropsychiatry Research Center (ONRC) of The Institute Of Living, Hartford Hospital, and with funding from UConn, and complimentary neuroimaging by ONRC. [See image at left for the diffusion tensor imaging results]
In Spring 2022, a modified version of the above study was completed as a single-subject pilot project to develop materials and methods. One individual with severe chronic TBI completed 40 hours of intensive evidence-based CRT. A robust set of treatment materials, and data collection methods, were developed. Additionally, programs in E-Prime were written to capture event related potential (ERP) measures using the Attention Network Test (ANT; Fan et al., 2009) and a cognitive switching task (Gajewski & Falkenstein, 2012). Qualitative interview was completed, and psychometric, and psychosocial data were also collected. The EEG/ERP work was completed in the electrophysiology lab of Dr. Brian Gonsalves, Dept. of Psychology, CSU East Bay.
Student research assistants at California State University East Bay (CSUEB) who worked on this project include: Mikhail Crosby, Jessica Myngheer, Chloris Li, Medha Lohani, Connie Roth, and Soujanya Konatalapalli. Jessica’s master’s thesis analyzed the qualitative interview results, and a manuscript of this is currently in review.
Several grants have supported this line of research. A $33K grant from the Firedoll Foundation provided student research scholarships, support for materials and participant compensation, and summer salary. The CSUEB Office of Research and Sponsored Projects and Center for Student Research provided funding to students and the PI.
An application to expand this line of research by incorporating functional Near Infra-Red Spectroscopy (fNIRS) has been submitted to the Switzer Fellowship program at the National Institute on Disability, Independent Living, and Rehabilitation Research (NIDILRR) in Spring 2024.
Metacognition – Cognitive Processes and Neural Substrates
Metacognition encompasses awareness/monitoring or controlling/regulating one’s own cognitive processes. This can be further subdivided by cognitive domain; for example, metamemory would be self-monitoring or self-regulation of one’s own memory processes. Metacognition is related to executive functions (EFs). EFs comprise a wide range of higher-level cognitive processes, such as reasoning, problem solving, planning, organizing, sequencing, etc. Research into executive function and metacognition has proceeded largely independently, particularly in terms of their hypothesized constituent cognitive processes and the manner in which these have been measured. Research in executive function typically employs psychometric tests to evaluate such cognitive processes as verbal fluency, working memory, sequencing, switching, inhibitory control, error detection, planning, and organization. Conversely, metamemory research employs a variety of empirical measures such as Feeling of Knowing, Judgments of Learning, and Retrospective Confidence Judgments.
Our research seeks to investigate and constrain theories on the neural substrates and cognitive mechanisms underlying Judgments of Learning (or JOLs). JOLs are the judgments people make about what they have learned. When students preparing for an exam consider what will be on the exam, and which topics they feel they need to study more (vs. those topics they feel they already have already learned well and can therefore study less), this is an example of making a judgment about one’s learning. This area of our research investigates the cognitive processes underlying metacognitive Judgments of Learning (JOLs), in neurotypical controls and individuals with TBI.
Our first publication found evidence that JOLs may be influenced by implicit memory factors; antipriming in particular seemed to reduce overconfidence in learning in individuals with TBI, while neurotypical participants remained well calibrated (i.e., not over-confident). See Ramanathan, Kennedy, & Marsolek (2014). The study was limited by a small sample size and in that implicit stimuli were only presented immediately prior to memory encoding. This left open the question as to whether implicit processes can directly influence the processes involved in JOL formation.
Therefore, in a follow-up study with my former UConn doctoral student Dr. André Lindsey (now an Assistant Professor at Nevada State University), we have collected data investigating the role of implicit memory in JOLs. In particular, the first experiment provided subliminal masked prime and antiprime stimuli immediately prior to encoding of cue-target word-pair stimuli, while the second experiment provided the implicit stimuli immediately prior to making a JOL. We found no effects from the implicit manipulations. A manuscript of this work is under revision as of summer 2024. Student research collaborators include two former doctoral students at UConn (Dr. André Lindsey, and Deb Brom), undergraduate research assistants at UConn (including: Evan Shuris, Jenine Entwistle, Jillian Kowalski, Rosamaria Didiano, Disha Patel), and at California State East Bay (Theresa Jingyun Yao). This research has been made possible by a faculty start-up grant at UConn, and generous research grants for faculty from the Office of Research and Sponsored Projects, California State University, East Bay.
A third study (Ramanathan, Liu, Chen, & Kennedy, 2022) sought to identify the cognitive processes underlying JOLs in neurotypical individuals, and how those processes might differ in individuals with moderate or severe TBI with good recovery of metacognition. As predicted by our cognitive processing model, we found that short-term memory and a variety of executive functions (inhibition, switching, and cognitive fluency) was associated with immediate JOL in neurotypical individuals, while long-term memory alone was associated with accurate delayed JOLs. However, in individuals with good recovery from moderate or severe TBI, immediate JOL accuracy was predicted only inhibition, and delayed JOL accuracy was only associated with switching. Yet both groups were equally adept at making JOLs. This suggests functional reorganization in individuals with moderate or severe TBI who have experienced good recovery of metacognitive processing. This research was conducted in collaboration with Dr. Ming-Hui Chen, department of statistics at the University of Connecticut, and (then) doctoral student Dr. Ran Liu, now at AbbVie laboratories.
Finally, to investigate the neural substrates of JOLs, functional MRI data have been collected, in conjunction with Dr. André Lindsey (Nevada State University) and Dr. Michael Stevens (Olin Neuropsychiatry Research Center, Hartford Healthcare Institute of Living. Here participants carried out a JOL task (memorizing cue-target word pairs and making predictive judgments of the likelihood that they will later recall the target words if given just the cue word) while in an MRI scanner. Data have been collected, and data analysis for this project is expected to begin in late 2024.
Computational modeling of Implicit Memory
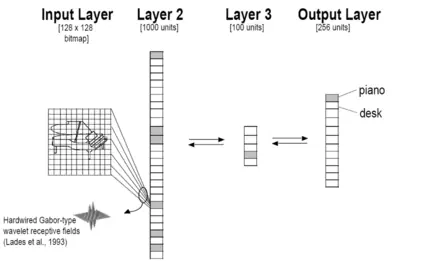
A visual object identification task has been instantiated in an artificial neural network, following the paradigm of Marsolek, Deason, Ketz, Ramanathan, Bernat, Steele, Patrick, Verfaellie, & Schnyer (2010), in order to examine whether objects need to be sufficiently similar for antiprimimg to occur, and whether the identification errors that are produced when a test object has been antiprimed can be predicted by neurocomputational models of the object identification system. The goal is to more clearly characterize why antipriming accompanies repetition priming.
This work is in collaboration with Drs. Rebecca Deason and Carmen Westerberg of Texas State University, Dr. Nicholas Ketz (University of Colorado), and the late Dr. Chad Marsolek (University of Minnesota).
Neurovascular Coupling
A new line of research, to begin in 2025, will involve using combined electroencephalography (EEG) and near infrared spectroscopy (NIRS) to investigate relationships between neuronal activation (as measured by EEG) and local vascular perfusion (as measured by NIRS). Thanks to generous funding by Texas State University, the BRAIN lab has acquired a NIRS system. This will permit measurement of training-induced functional neuroplasticity using both neuroelectric and hemodynamic modalities in resting state and task-related conditions.
